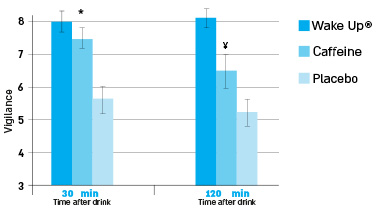
Higher performance on all tests was seen at 30 minutes for WakeUp!® compared to caffeine and placebo (P<0.05 for all), [MWS1] however, after 120 minutes WakeUp!® performance remained high compared to placebo, but caffeine performance decreased significantly on all tests compared to WakeUp!® (all p<0.05) There were no differences in heart rate or blood pressure for WakeUp!® consumption.Read Study
The Science

Dr. Giora Pillar
Head Researcher
Giora Pillar, MD, PhD, conducted three clinical studies on BioLift’s ™ WakeUp!® formula at The Technion-Israel Institute of Technology in Haifa, Israel, where he is a professor of medicine and an internationally recognized authority on Chronobiology and the field of sleep. Dr. Pillar received his medical and scientific degrees from the Technion, then completed a fellowship at the Sleep Medicine, Endocrinology, and Circadian Rhythm Department of Brigham & Women’s Hospital and Harvard Medical School. He also heads the sleep clinic at Clalit Medical services in Haifa and is certified by the American Board of Sleep Medicine. Dr. Pillar has over two decades experience in diagnosing and treating patients with sleep disorders and in teaching courses on sleep medicine. The author of more than 100 papers in peer review journals and a definitive handbook for clinicians on sleep disorders used worldwide, he has received numerous honors for his work.

Second clinical study: Longitudinal open label prospective study to assess the efficacy and tolerance of daily usage of the wake promoting beverage WakeUp!® .
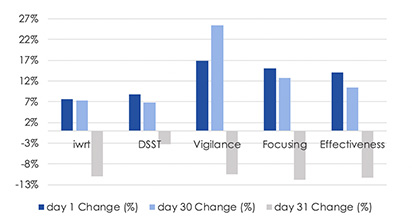
Performance on vigilance, concentration and Digit Symbol Substitution Test (DSST) all improved 1 hour after consumption of WakeUp compared to before use on Days 1 and 30 (all p<0.05). No tolerance over time was observed.
On Day 31, performance on vigilance, concentration, work performance, immediate Word Recall Test (iWRT) parameters all decreased one hour following lunch (p<0.01), providing additional proof-of-concept data DSST significantly improved over the course of 30 days with a plateau instead of a reduction on day 31, suggesting that consistent ingestion of WakeUp may improve brain function (processing speed) over time Read Study
Open label study assessing the efficacy of one bottle of WakeUp!® in the morning (8:30 am) and one in the late afternoon/evening (6:30pm) of the same day before and 30 minutes after drink. Testing done before and 30 minutes after consumption. Outcome measures included Objective iWRT, DSST Standard subjective scale for vigilance, focus and work performance. Read Study Read Study
.png)
The fourth clinical study: Complementary open label
.png)
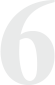
Patents:

US Trademark No. 86138675 in Classes 50 and 32 in the name of InnoBev Ltd.
WAKEUP! POST LUNCH WAKER | WAKEUP! BIO-WAKER
Approved Claims
According to the clinical studies done with WakeUp!® formula, the following suggested structure-function claims may be used in the USA only as follows:


